Doris Kra
05/03/2019
Chemistry
Miss. Block
What is a combustion reaction
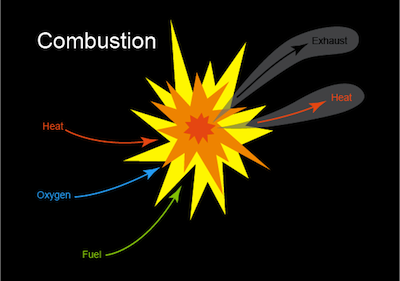
(b)
Some examples of a Combustion reaction are:
05/03/2019
Chemistry
Miss. Block
What is a combustion reaction

(b)
Some examples of a Combustion reaction are:
- Combustion reaction of Methane CH₄(g) + 2 O₂(g)→ CO₂(g) + 2 H₂O(g)
- Combustion reaction of Propane 2 C₃H₈(g) + 7 O₂(g) → 6 CO₂ (g) + 8 H₂O(g)
- Combustion reaction of Ethane 2 C₂H₆ + 7 O₂→ 4 CO₂ + 6 H₂O
Citations
a) https://www.google.com/search?q=combustion+reaction&rlz=1CAKSOU_enCM687CM716&source=lnms&tbm=isch&sa=X&ved=0ahUKEwjZkLTA4-vgAhVbRhUIHcV6AM0Q_AUIDigB&biw=1300&bih=572#imgrc=08oruGJQRM7f7M:
b) https://media1.shmoop.com/images/chemistry/chembook_stoich_graphik_22.png
Comments
Post a Comment